Key findings
- Japan Tissue Engineering (J-TEC)’s autologous cartilage repair product (JACC) is now reimbursed in Japan for knee osteoarthritis.
- Coverage is for patients who have not improved with conservative therapy and have a cartilage defect of at least 2 cm².
- In a 27-patient study, J-TEC reports WOMAC scores improved more than with sodium hyaluronate injections, with no safety signals of concern observed.
Japan Tissue Engineering Co., Ltd. (J-TEC) reports that Japan’s National Health Insurance system has approved reimbursement of JACC, the company’s autologous cultured cartilage product, for a new indication: knee osteoarthritis.
The reimbursement took effect January 1, 2026, and it’s the first therapy in Japan to receive manufacturing and marketing approval and national insurance coverage for this indication.


Japan has an interesting legal framework for bringing regenerative medicine therapies to market faster than waiting for full Phase 3 trials and approval. You can read our interview with JSRM, the non-profit behind the laws, here.
KOA Problem
Knee osteoarthritis is a progressive condition where cartilage wears down or becomes damaged (often due to aging or injury), leading to pain, joint deformity, and reduced mobility. J-TEC estimates that about 10 million people in Japan have knee osteoarthritis, and up until now, there haven’t been many effective therapies to directly treat the cartilage itself.
What’s JACC?
JACC is a gel-like “artificial cartilage” made by culturing chondrocytes (cartilage cells) taken from a patient’s normal knee cartilage. It was invented in 1996 by Mitsuo Ochi (President of Hiroshima University), and under his supervision, the company has led development for manufacturing and marketing since 2000.
Regulatory timeline and use to date
J-TEC says JACC was first approved in July 2012 for traumatic cartilage defects and osteochondritis dissecans of the knee, and later received an additional approval for knee osteoarthritis in May 2025. Since its April 2013 launch, J-TEC reports JACC has been used in more than 2,000 cases of traumatic cartilage defects or osteochondritis dissecans of the knee (excluding osteoarthritis).
Who qualifies for reimbursement, and how the procedure works
According to the company, the new national insurance reimbursement applies to knee osteoarthritis patients who:
- have not improved with conservative therapy (for example, exercise therapy), and
- have a cartilage defect area of at least 2 cm²
JACC is transplanted into the cartilage defect and covered with a fixation membrane (either an artificial collagen membrane or periosteum) that is sutured to the surrounding tissue. J-TEC states this targets patients with moderate knee osteoarthritis who have sufficient cartilage around the defect to allow suturing.
Clinical study results
- Efficacy: Improvements in WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) from pre- to post-treatment were superior to sodium hyaluronate injections.
- Safety: No adverse events of concern were observed.
- Cartilage repair (secondary endpoint): At 52 weeks post-treatment, the implanted site was confirmed to be repaired with tissue similar to normal cartilage.
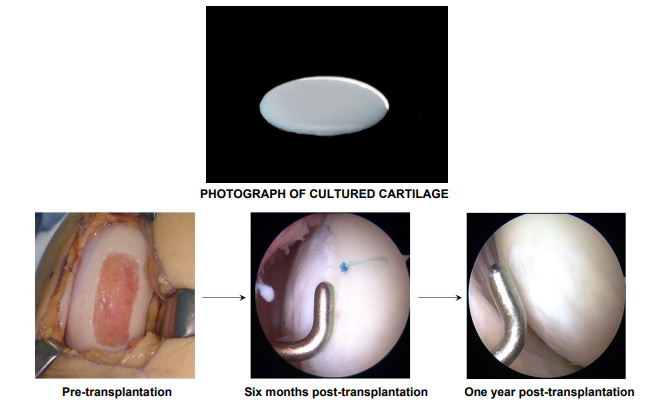
J-TEC says that because JACC uses a patient’s own cells, the risk of immune rejection is extremely low.
Looking ahead, J-TEC plans to strengthen information dissemination about JACC and ensure a stable supply, with the goal of delivering treatment to about 1,000 patients annually within a few years.
“As Japan’s population ages, treating knee osteoarthritis has become a major societal challenge.,” said Kazuto Yamada, President of J-TEC. “Delivering JACC to osteoarthritis patients has been our longstanding aspiration. With reimbursement approval now in place, together with stable supply and support for appropriate use provided by J-TEC, access to JACC will expand. This first-in-Japan therapy will contribute to a better quality of life for more patients.”
More information: https://www.jpte.co.jp/en
Want to keep up on regenerative medicine? Get the weekly newsletter here.


















