South Korea’s MEDIPOST, Inc., a company developing stem cell-based therapeutics, has announced the appointment of five senior executives, which they say will bring decades of expertise in biologics and cell & gene therapy. This development is part of the company’s efforts as it introduces its regenerative therapy, CARTISTEM®, to patients in the U.S. and aims to address degenerative diseases.
CARTISTEM is reported to be the world’s first regulatory-approved allogeneic human umbilical cord blood-derived mesenchymal stem cell product. Initially launched in South Korea in 2012 for treating knee osteoarthritis (OA), the company mentions the product boasts over 10 years of real-world clinical data and has been used in more than 30,000 patients.
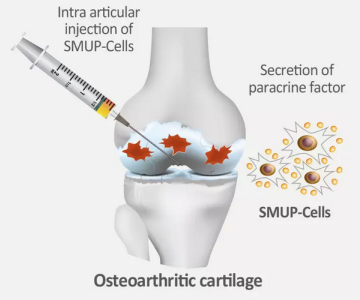
The U.S. team’s immediate goal is to secure Investigational New Drug (IND) approval to initiate a Phase III clinical trial, facilitating a Biologics License Application (BLA) and a commercial launch in North America.


The executive appointments follow MEDIPOST’s manufacturing partnership with OmniaBio and the opening of OmniaBio’s cell and gene therapy facility in Ontario. MEDIPOST’s collaboration with OmniaBio is set to leverage expertise in cell and gene therapy manufacturing and analytical technologies, crucial for CARTISTEM’s scale-up and distribution.
The new executives joining MEDIPOST include:
+ Adrian Orr, Head of Clinical Development, brings 25 years of experience in osteoarthritis and cartilage repair, focusing on clinical trials for CARTISTEM’s U.S. debut.
+ Jagannadha Rao Kandula, Ph.D., Head of CMC & Operations, has over 25 years in scaling biologics and cell therapies, with previous roles in cell therapy approval and commercialization.
+ Keith Bentlage, Head of Project Management Office, has led programs to successful Phase III trial completion and commercial launch for over 30 years.
+ Richard Ong, Head of Quality, offers extensive experience in quality control from early to late-phase programs, ensuring CARTISTEM meets high standards through commercialization.
+ Raymond Bergeron, Head of Accounting & Controller, has over 20 years in financial leadership, focusing on efficient operations and strategic growth.
MEDIPOST, Inc. focuses on developing umbilical cord-derived stem cell therapies for treating inflammation-driven degenerative diseases. The company’s flagship product, CARTISTEM, is the first allogeneic stem cell therapy for knee osteoarthritis (OA), launched in Korea in 2012 and used in over 30,000 patients. Following its U.S. Phase 1/2a clinical trial, MEDIPOST is preparing its IND for a Phase III study. In collaboration with the Centre for Commercialization of Regenerative Medicine (CCRM), MEDIPOST has established OmniaBio in Hamilton, Ontario, for CARTISTEM’s manufacturing.