Key findings
- Umbilical-derived stem cell exosomes reduced inflammation and promoted cartilage regeneration in both human and mouse models
- Demonstrated safety and tolerability in a randomized clinical trial
- Some improvement in clinical symptoms of osteoarthritis was seen post-treatment
- Needs further research
Exosome Therapy Shows Promise for Osteoarthritis: From Bench to Bedside
Osteoarthritis (OA) is a major cause of disability worldwide, driven by chronic inflammation and the progressive breakdown of joint cartilage. Traditional treatment options are limited, often failing to restore cartilage or halt disease progression. A recent study led by researchers in China explored a novel approach: using exosomes – tiny vesicles produced by human umbilical cord mesenchymal stem cells (hUC-MSCs) – to treat OA.
Mesenchymal stem cell-derived exosomes (MSC-Exos) have properties similar to stem cells themselves but are believed to be more stable and less likely to trigger immune rejection. The team began by isolating hUC-MSCs from donated umbilical cord tissue, confirming their ability to become bone, cartilage, or fat cells in the lab. Exosomes were then extracted and thoroughly characterized, showing typical markers and morphology expected of these nano-sized vesicles.


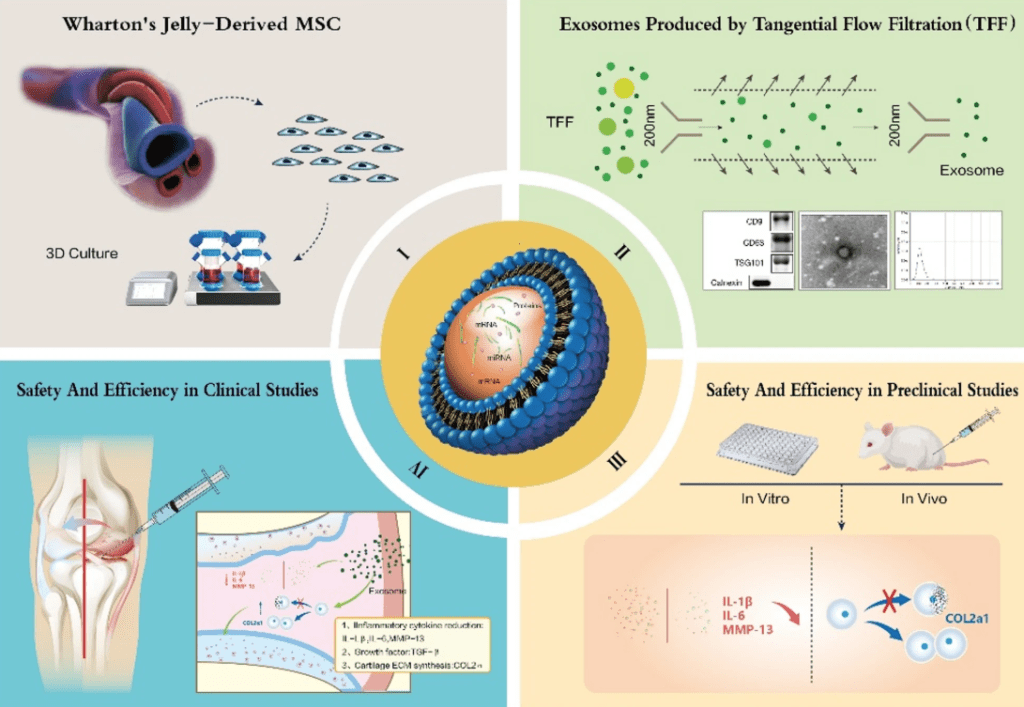
Image Credit – from the research published in Journal of Translational Medicine
Laboratory and Animal Evidence of Regeneration
The researchers first tested the hUC-MSC-Exos in laboratory settings. Mouse chondrocytes (cartilage cells) treated with these exosomes showed improved survival and proliferation. When inflammation was artificially induced using IL-1β, a cytokine involved in OA, exosome treatment reduced the expression of inflammatory molecules (such as IL-6 and MMP13) and increased levels of type II collagen, a protein essential for healthy cartilage.
Next, the team moved to a mouse model of OA. After chemically inducing OA in the knee joints of mice, they injected hUC-MSC-Exos into the affected joints. The treated animals showed less cartilage damage, smoother joint surfaces, and more normal cartilage structure than untreated OA controls. Histological analysis found more collagen and fewer markers of cartilage breakdown in the exosome group. These results were consistent with the improvements seen at the cellular level.
Early Clinical Assessment in Human Patients
To investigate whether these findings would translate to humans, the researchers performed safety tests on human chondrocytes and then initiated a randomized, double-blind, dose-escalation clinical trial. Forty-one OA patients received three intra-articular injections of hUC-MSC-Exos over six weeks, at one of three different doses.
None of the patients experienced significant adverse effects, and laboratory measures of liver and kidney function remained stable throughout the nine-month follow-up. In terms of efficacy, the trial assessed pain, stiffness, and functional ability using the WOMAC scoring system. Improvements in pain and function were observed in different dose groups, with the highest dose group showing the most consistent benefits over time.
MRI scans in some participants revealed reduced joint swelling, decreased effusion, and, in some cases, thickening of the cartilage after treatment. While these results suggest that hUC-MSC-Exos may reduce inflammation and promote cartilage repair in OA, the authors note several limitations:
The clinical trial was relatively small and did not include a placebo control group. Most patients had mild-to-moderate OA, so the effects in severe cases are not yet known. Imaging follow-up was not completed for all participants due to resource constraints. Despite these limitations, the study provides early evidence that hUC-MSC-derived exosomes could offer a new, cell-free approach to OA treatment. Larger, controlled trials and further mechanistic studies will be needed to clarify optimal dosing and long-term outcomes.
Want to keep up on regenerative medicine trials? Get the weekly newsletter here.
Wang, Y., Kong, Y., Du, J. et al. Injection of human umbilical cord mesenchymal stem cells exosomes for the treatment of knee osteoarthritis: from preclinical to clinical research. J Transl Med 23, 641 (2025). https://doi.org/10.1186/s12967-025-06623-y