About a decade ago, Japan changed how it regulates regenerative medicine. They now allow the use of cell & gene therapies before they’re fully approved, under certain conditions.
The regenerative medicine framework is split into two pathways:
- PMD Act – For manufacturers seeking full market authorization
- ASRM – For physicians seeking to use cell & gene therapies for their patients/trials


That system has fueled rapid access and pioneered discoveries, but also created loopholes and drawn criticism along the way.
Recently, the FDA has shown smoke signals that it may change our regenerative medicine laws. For instance, in March 2025, Dr. Peter Marks, former head of CBER, said they were reevaluating the regenerative medicine framework. Since then, the FDA has held a variety of public meetings on this topic, and Florida recently followed suit with Utah and other states, allowing birth tissue stem cells under certain conditions.
As we move through these changes, what can we learn from Japan’s regulatory experiment?
To find out, we sat down with the non-profit with a deep history of shaping not only the regulatory pathways but also the science itself.
Here’s the interview with the Japanese Society for Regenerative Medicine’s Secretary General, Kyosuke Mano, who details why the laws were made, how they work, lessons learned along the way, and the additional frameworks they’re working on to improve that system.

*Quick clarification – some of these acronyms are very similar, which can be confusing.
- The PMDA is the Japanese equivalent of the FDA (The Pharmaceuticals and Medical Devices Agency); it’s their governing body.
- The PMD Act, on the other hand, is the new regenerative medicine pathway, which is implemented by the PMDA.
Additionally, Japan has a national insurance system.
Enjoy the interview!
Who is JSRM?
Mano: JSRM began in 2001, pivoting from a collective of hematologists called The Cell Therapy Research Group. We’ve not only advanced the science of regenerative medicine but also helped shape the regulatory system along the way. We also run our own peer-reviewed journal on the topic.
A few examples of our scientific advancements:
- Former JSRM president Professor Teruo Okano invented temperature-responsive culture dishes, paving the way for cell sheet engineering.
- His successor, Professor Yoshiki Sawa, developed the world’s first skeletal muscle-derived cardiomyocyte sheets, demonstrating the real potential of regenerative therapy for heart disease.
- After the discovery of induced pluripotent stem cells (iPSCs), our then-president, Professor Hideyuki Okano, shifted the focus, developing iPSC-derived neuro-progenitor cells for spinal cord injury. This marked a new frontier and established Japan as a global leader in the industry.
Throughout these discoveries, we’ve worked closely with the PMDA and Medical Devices Agency to design and implement the two-track system.
The Global Issue: Regenerative Medicine Doesn’t Fit Pharmaceutical Standards
Mano: Prior to 2014, depending on their modality, regenerative therapies were regulated under existing pharmaceutical or medical device laws, even though their characteristics didn’t exactly match. (Sound familiar?)
For instance, tissue-engineered products such as Japanese Autologous Cultured Cartilage (JACC) and Japanese Autologous Cultured Epidermis (JACE) were regulated as medical devices.
To solve this, we helped the government design the PMD Act, creating a novel pathway for the mass-manufacturing and marketing of these therapies at scale.
Additionally, Japan has a long-standing history of physician independence, often allowing leeway to administer unapproved therapies at their discretion, without government oversight. Unfortunately, this gives little visibility into what exactly is being administered, how it’s being done, and its efficacy.
So, in addition to the PMD Act, we introduced ASRM to bring transparency and accountability to the clinic. We still allow administration of cell and gene therapies outside of clinical trials, but they must be reviewed by accredited committees and registered with the Ministry of Health, Labor and Welfare.
This enables real-time monitoring and grants the government power to intervene when necessary, without lengthy court cases.
We have strictly defined standards, similar to the FDA’s:
- PMD Act therapies must adhere to Good Gene, Cellular, and Tissue-based Products Manufacturing Practice (GCTP) – similar to current Good Manufacturing Practice (cGMP).
- The trials are regulated under Good Clinical Practice (GCP), which is aligned with ICH GCP (E6) guidelines for ethical and scientific conduct.
- ASRM requires the CDMOs (known as CPFs, or Cellular Processing Facilities in Japan) to follow GCTP, though in a more limited form.
- We also require the ethical conduct of GCP, rather than both the ethical and scientific conduct, like the PMD Act does.
In short, though similar, ASRM has a lower threshold than the PMD Act.
While this system has enabled Japan to rapidly advance the industry and science, it has also drawn criticism, such as:
- Limited capacity to review efficacy
- Potential conflicts of interest
- Loopholes permitting, sometimes, arbitrary treatments
- An unusually low number of reported adverse events
These issues highlight the challenge of balancing patient access, innovation, and safety in a framework that governs both industry and private clinical practice.
Here’s an infographic on how those laws work:

ASRM – The Physician Pathway
In short, ASRM allows physicians to administer unapproved therapies at their discretion, but they must seek and receive positive comments from government-accredited committees first and follow the guidelines above.
There are separate “classes” of therapies, based on their risk profiles, which determine how difficult the approval process will be. If you’re familiar with the HCT/P laws in the USA, imagine a 351A, B, and C track, with varying difficulty of approval.
Regen Report: One global issue is that every advancement seems to hold promise, but also results in a regulatory “edge case”. The nuance is difficult for both industry and regulators to interpret: how do we regulate this?
How does Japan deal with that?
Mano: ASRM divides these therapies into one of three classes, based on their risk profile. Therapies in Class 1 have the most stringent review process, while Class 3 has the least. Keep in mind, not every regenerative therapy fits into ASRM, such as extracellular vesicles (EVs); this is for cellular and gene-based therapies only.
Here’s how those classes work:

CLASS 1 (Most Stringent Approval)
- Anything pluripotent-derived. This would include induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs)
- Anything genetically modified. So gene therapy, CAR-T, TCR-engineered immune cells, Nurexone Biologic’s exosomes loaded with siRNA, etc.
- Anything allogeneic. This includes all stem cells derived from birth tissues, adipose, bone marrow, etc., regardless of in vitro culture.
- Xenogenic, derived from a species other than humans.
CLASS 2
- Autologous cells with in vitro culture, such as expansion, whether from your bone marrow, adipose, or preserved birth tissue.
- For instance, NurOwn is an autologous, bone marrow-derived mesenchymal stem cell (MSC) therapy with increased neurotrophic factors. If this were used in a physician-led setting, this would likely fit into Class 2.
- Any cells used outside of homologous use* that do not fit into Class 1.
CLASS 3 (Least Stringent Approval)
- Cells that are autologous, minimally manipulated, and considered homologous use*.
Regen Report: *Homologous use can be a significant point of contention. How does the PMDA define this? Could you give examples?
Mano: According to the PMDA, “homologous use” refers to administering cells in a way that matches the original function of those tissues or cells.
For example, harvesting adipose tissue from the abdomen, isolating MSCs, and using them for breast reconstruction after mastectomy is considered homologous use.
In contrast, intravenous administration of those same cells for diabetes is not homologous use.
Speaking on your example, most WJ products are acellular and would be outside of the scope of ASRM; however, if they use WJ as a source for MSCs, yes, the original biological role of WJ is to cushion the umbilical cord, and it could be argued that cushioning a joint is homologous use. If the WJ is autologous, the cells are not expanded, and it’s used in a homologous way, it could potentially fit into Class 3. However, expanding those would cross into Class 2, and if they are allogeneic (regardless of expansion), or used for purposes that aren’t homologous, that lands in Class 1.
It is the responsibility of the physician to correctly designate the class in their provisional plan, and the accredited committee reviews the appropriateness of that classification, along with the clinical plan, for potential approval. They have final say.
Depending on the accredited committee’s schedule, the time from submission to implementation is about 3 months for Classes 2 and 3. Class 1 requires an additional review by the Health Science Council, which can take longer. That council meets monthly, and approvals rarely occur in a single review round.
Regen Report: Florida’s new stem cell law has specific requirements for cell viability. Does Japan have something similar?
Mano: ASRM does not mandate third-party validation for issues such as cell viability, so unfortunately, there is no guarantee that all cells remain viable at the time of administration.
In Japan’s private practice market, some manufacturers even bypass ASRM criteria by offering cell-free formulations, such as lyophilized culture supernatant products, thereby avoiding accredited committee review and government oversight altogether.
Regen Report: Hypothetically, if the company discontinues PMD Act clinical trials, are physicians allowed to use them in the ASRM track?
Mano: Yes, this happens. Some companies are now considering strategies to offer treatments under the ASRM framework, drawing on data accumulated from discontinued clinical trials as supporting evidence.
As long as the data is collected in compliance with Good Post-marketing Study Practice (GPSP), companies can use ASRM data to apply for the PMD Act as well. However, the ASRM framework does not require registration in GPSP-compliant registries, so regrettably, many clinics provide treatments without systematically gathering scientific outcome data.
PMD Act – The Manufacturers’ Pathway
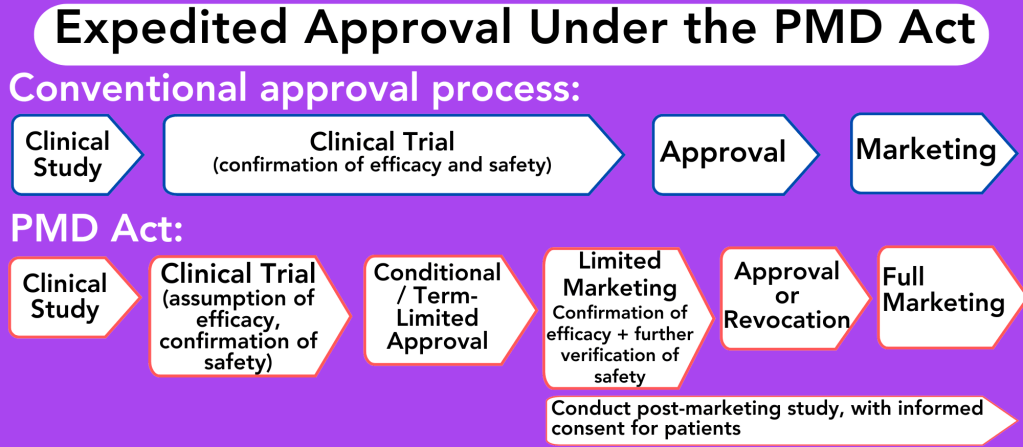
In short, if a therapy shows promise in early trials, the PMD Act allows these therapies to enter the market with time limits, while post-marketing surveillance is conducted to confirm safety and efficacy.
Mano: In JSRM’s 2012 Yokohama declaration, we urged regulators to allow conditional approval if safety is ensured at the review stage, provided that high-quality post-marketing surveillance is in place.
This system enables faster patient access to novel therapies by permitting approval based on surrogate endpoints, subject to specific conditions and a time limit. Products, even those in the early conditional and time-limited approval stages, are covered under our national insurance program, which reimburses approximately 70% of the cost. ASRM therapies, by contrast, are not typically covered.
During conditional approval, we regulate how companies market these therapies. They must inform patients that efficacy is not proven, and patients will need to participate in post-marketing surveillance.
Manufacturers are required to collect the clinical outcome data, which we use to determine if full approval should be granted after the conditional period.
To foster that post-marketing surveillance, JSRM developed a third-party registry, called NRMD/PMS, which meets the regulatory quality standards. However, at this time, use of the registry is not legally mandated, and uptake has been limited. Many companies still use post-marketing data by way of in-house registries, often lacking the independence of a third-party system. We’re still facing that challenge.
On a positive note, we have a variety of therapies that have gone through the PMD process and achieved full market approval, and a few are currently in conditional approval as we speak:

ASRM Improvements: New Sub-Framework
Mano: ASRM has been great at expanding access, but unfortunately, the outcomes again are often internal, unstandardized data, making it difficult to evaluate efficacy and obtain insurance coverage.
To mitigate this, we’re developing what we call the Explorative Therapy framework, which adds another layer to ASRM. The primary goal is to collect, define, and unify the data while strengthening informed consent and ensuring that, before treatment, sufficient evidence based on preclinical and clinical evaluations is established, so the science can progress safely.
We’re proposing to classify ASRM therapies into two categories, Explorative Therapies and Uninvestigated Therapies, to provide a clear ethical and scientific framework, not to create division:
- Explorative Therapy: Therapy using processed cells or nucleic acids, etc., for which a manufacturing and marketing approval has not been obtained under the PMD Act, and clinical data are accumulated in an independent third-party registry, and an Explorative Study is conducted both prior to and following the treatment
- Explorative Study: A study conducted by a physician (or dentist) to scientifically confirm the safety and efficacy of regenerative medicine therapy that he or she provides or has provided, by quantitatively analyzing the clinical data of the therapy
- Uninvestigated Therapy: Therapy not qualified as Explorative Therapy, by using processed cells or nucleic acids, etc., for which a manufacturing and marketing approval has not been obtained under the PMD Act. (Essentially, those not participating in the above Explorative Frameworks).
“The intent is to grant physicians ethically bounded freedom while requiring pre-treatment evidence based on preclinical or in-vitro/in-vivo assessments, and to generate transparent, cumulative evidence for patients, policymakers, and doctors.”
Outcome data can be difficult to standardize because, for example, neurodegenerative disorders are quantified very differently from orthopedics. One of the registries for this new framework is developed entirely in-house by JSRM, for which we worked directly with relevant specialty societies, like the Japanese Orthopedic Association, to harmonize data collection from individual domains.
We’re currently building the OAK control dataset, a public, standardized orthobiologics registry, as that’s a very common use case for regenerative medicine. Beyond facilitating patient-informed decision-making, this registry aims to establish a universal historical control group: a scientifically robust reference dataset that allows consistent comparison across diverse regenerative and orthobiologic therapies.
By standardizing pre-/post-imaging, pain/function scores, and other clinical data, the OAK control dataset will not only enhance transparency but also serve as a foundation for future clinical validation and policy-making in orthopaedic regenerative medicine.
What else is in the pipeline for JSRM?
This interview has barely scratched the surface of what we do; we have a variety of other projects in the pipeline as well.
For instance:
We’re accelerating iPSC-derived therapies by co-hosting new significant events:
- Joint International Conference on Clinical iPSCs with ISCT in Kobe (March 2026)
- iPSC 20th Anniversary Symposium with ISSCR and CiRA in Kyoto (October 2026)
Recently, we began a Pan-American partnership with the Alliance for Regenerative Medicine (ARM) in the USA. Together, we’re discussing the possibility of making ARM’s Cell and Gene Meeting series more accessible to stakeholders in Asia, the Middle East, and lower-income countries. This is important because the knowledge and cultural acceptance of regenerative therapies in Asia differ from Europe and North America, and the programming must reflect the local communities.
The ultimate goal? We’re building a global ecosystem that fosters science while delivering safe, efficacious therapies, so the world can live healthier, happier lives.
We have a long road ahead, but so far, I’m proud of our accomplishments not only as an organization, but as a country.
Special thanks to Kyosuke Mano for the interview!