Aspen Neuroscience has closed a $115 million Series C financing round to continue development of its lead stem cell candidate, ANPD001, for moderate-to-advanced Parkinson’s disease.
ANPDD001 is an autologous iPSC-derived dopaminergic neuronal precursor cell (DANPC) therapy, and it’s currently in Phase 1/2a trials. They mention they’ve developed a proprietary delivery system that combines metered-dosing syringe technology with MRI guidance, enabling sub-millimeter accuracy during transplantation.
With this latest round, including an $8 million grant from the California Institute for Regenerative Medicine (CIRM), Aspen’s total capital raised now exceeds $340 million. They’ll also gain a new board member, Cindy Perettie, Executive VP from one of the funding partners, Kite.


Plans for Funding:
- Support ongoing Parkinson’s trials
- Expand manufacturing capabilities
- Advance additional autologous iPSC-derived therapies targeting other neurological conditions
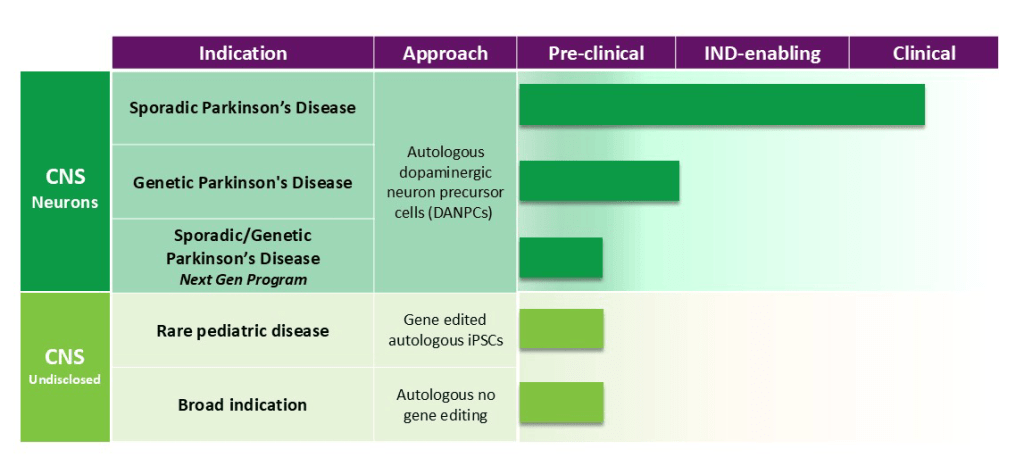
Here is the pipeline according to their website:
Aspen also mentioned that it has developed a proprietary manufacturing platform that uses machine learning combined with advanced genomics to produce high-quality personalised cells for each patient, and they’re aiming to improve consistency at a larger scale.
Recent Progress
- Cohort 3 dosing has started in the ASPIRO Phase 1/2a trial, which now uses a commercial-ready formulation of ANPD001. According to preclinical studies, this formulation is comparable to those used in earlier cohorts.
- This new formulation is cryopreserved, ready-to-dose, and will hopefully simplify workflows for hospital labs.
- Previously reported six-month data from earlier cohorts showed strong safety/tolerability, along with both clinician- and patient-reported improvements, all without immunosuppressive drugs (as opposed to allogeneic cells, which may cause an immune response)
For further details, visit their website: www.aspenneuroscience.com.
Want to keep up on regenerative medicine? Get the weekly newsletter here.















