Key findings
- MEDIPOST has filed an IND with the FDA to start a Phase 3 trial of Cartistem for knee osteoarthritis.
- The planned Phase 3 is randomized, multi-country, and uses an active control in patients with moderate-to-severe disease.
- In Japan, MEDIPOST has finished the final patient visit in its Phase 3 trial and expects results in H1 2026.
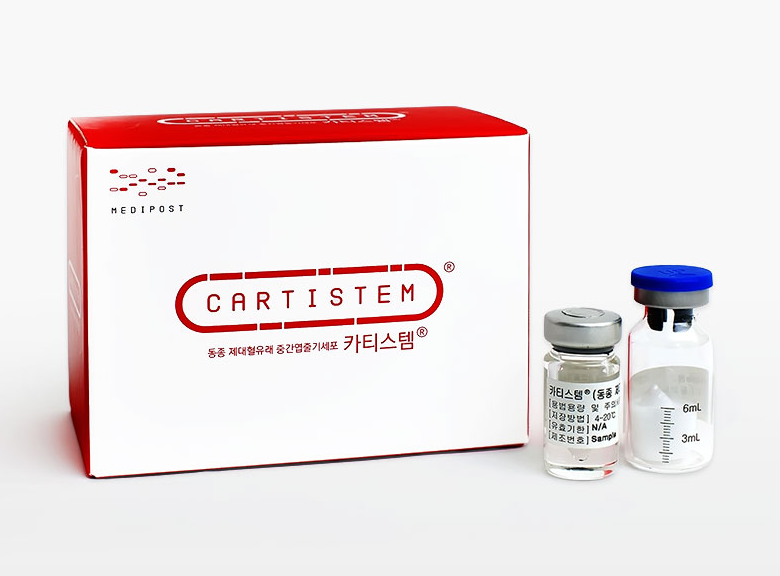
MEDIPOST has submitted an Investigational New Drug (IND) application to the FDA for Cartistem, its allogeneic (donor-derived) umbilical cord blood-derived mesenchymal stem cell (MSC) therapy for knee osteoarthritis.


Publisher note (Jeremy) – I’ve been telling people about Cartistem for years; it’s a very exciting candidate for KOA, yet not many folks in the West know about it… yet.
In 2012, I believe it became the world’s first approved umbilical cord blood product (note that it was only approved in Korea, where MEDIPOST is based). They’ve published a few studies with before/after arthroscopic images, MRIs, and post-administration biopsies to confirm that cartilage cells were generated. Based on this and their US Phase 1/2a results, the FDA gave them a Phase 2 waiver, and they’re now heading into Phase 3.
If that goes positively, it could do a few things. For one, most physicians still seem to be hesitant about orthobiologics due to the insufficient evidence, this could have an effect.
It may also address many “battleground” questions in orthobiologics, such as:
- How does an allogeneic MSC therapy stack up against platelet-rich plasma (PRP) and autologous bone marrow concentrate (BMAC)?
- If it’s neck and neck, would patients opt for a bone marrow aspiration or an off-the-shelf therapy?
- BMAC has very few stem cells. I’ve had two procedures, each yielded 1B total cells +/-. Of those total cells, the peer-reviewed literature says it’s about .01-.001% MSCs, meaning 10-100K MSCs. In one Cartistem study, researchers said this: “We question whether the term ‘stem cell therapy’ is acceptable for this kind of medical procedure [BMAC] containing only a small number of stem cells.” I believe Cartistem dosing is in the millions of MSCs. Could this lead to more significant outcomes?
- Are allogeneic MSCs cleared by the host’s immune system, or do they integrate and regenerate new tissue? This is a common criticism of allogeneic orthobiologics by their autologous counterparts.
U.S. plan: Phase 3 trial starting in 2026
The upcoming Phase 3 will reportedly be a randomized, multi-country study with an active control group to evaluate Cartistem’s efficacy and safety in patients with moderate-to-severe knee osteoarthritis. MEDIPOST plans to treat the first patient in the first half of 2026.
MEDIPOST says it intends to use the Phase 3 to support its structural-improvement claims tied to cartilage regeneration, with the goal of positioning Cartistem as a disease-modifying osteoarthritis drug (a therapy intended to change the course of the disease, not just manage symptoms).
The company expects it could obtain U.S. marketing authorization around 2031, followed by a commercial launch shortly thereafter.
Plan for Japan: Phase 3 just finished
MEDIPOST just wrapped up its Phase 3 in Japan. They said they’ll announce the results in the first half of 2026, then apply for marketing authorization in the second half, with a stated goal of entering the Japanese market in 2027.
What’s “marketing authorization” in Japan? Read our interview with JSRM, the non-profit behind Japan’s regenerative medicine laws, to learn all about Japan’s stem cell regulations. It’s an interesting system which allows therapies into the market before Phase 3 and full approval.
Following the Japanese trial completion, MEDIPOST signed an exclusive distribution agreement with Teikoku Pharma for which they MEDIPOST received an upfront payment of 11.8 billion won ($8.1 million) and is eligible for milestone payments of 14.8 billion won if it receives authorization in Japan.
Want to keep up on regenerative medicine? Get the weekly newsletter here.