The interest in stem cells is booming and it may become legal in the USA shortly… however, there’s a lot of conflicting information about the products, both from the companies selling them and the companies competing against them.
One thing that’s claimed by competing companies is that Wharton’s Jelly (the jelly in umbilical cords) has “no living stem cells” (at least the commercialized product), and it’sa fraud.
That “containing stem cells” or not is a topic for an entire series, but regardless, did you know that Wharton’s Jelly has a lot more than just stem cells?


This company sent their Wharton’s Jelly to a variety of third-party labs to get the exact composition, and wrote a study on it.
Here’s a breakdown of what they found.
Methods:
Human umbilical cords were obtained with informed consent from Caesarian-section donors, then screened and tested following FDA and American Association of Tissue Banks guidelines.
The researchers then isolated Wharton’s Jelly tissue and converted it into an injectable formulation without using digestive enzymes, cryoprotectants, or in vitro cell expansion. They also mentioned this formulation was minimally manipulated according to FDA guidelines, with no reliance on living cell metabolic activity (if they meet these guidelines, they are considered a 361 product).
The researchers conducted analyses on samples from various batches:
- 60 samples were tested for sterility.
- 6 samples were analyzed for growth factors and cytokines using a specialized growth factor/cytokine array.
- 6 samples were evaluated for hyaluronic acid content using an ELISA kit.
- 12 samples were screened for extracellular vesicles using nanoparticle tracking analysis, confirming their membrane-encapsulation (indicating true extracellular vesicles).
Results:
All tested samples passed sterility tests.
Researchers report the detection of significant amounts of growth factors, cytokines, hyaluronic acid, and extracellular vesicles:
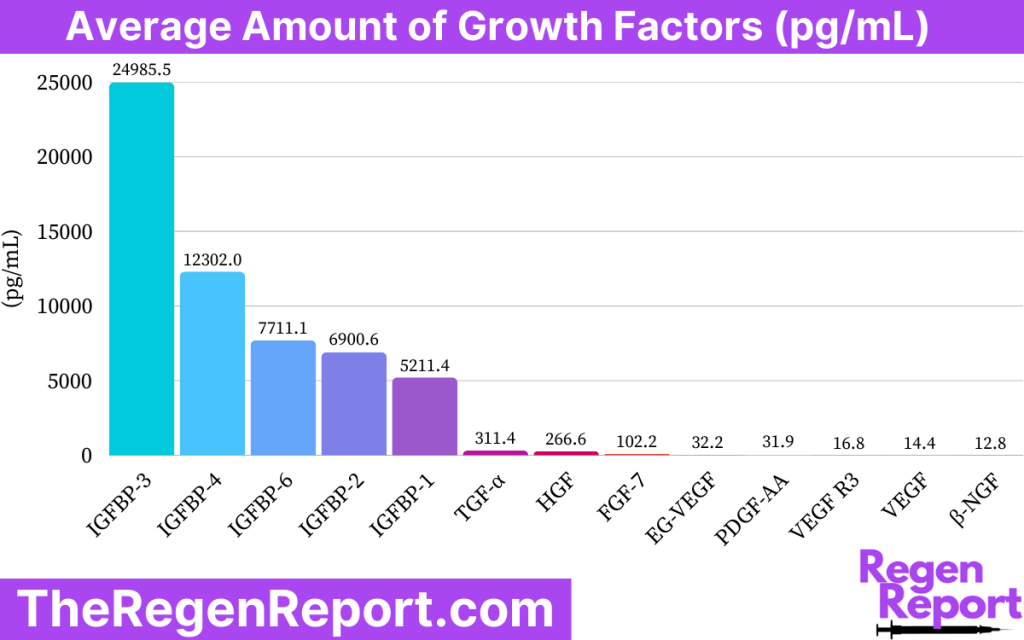
Growth Factors (average concentrations):
- Insulin-like growth factor binding proteins (IGFBPs): IGFBP-3 (24,985.5 pg/mL), IGFBP-4 (12,302 pg/mL), IGFBP-6 (7,711.1 pg/mL), IGFBP-2 (6,900.6 pg/mL), IGFBP-1 (5,211.4 pg/mL)
- Transforming growth factor alpha (TGF-α): 311.4 pg/mL
- Platelet-derived growth factor AA (PDGF-AA): 31.9 pg/mL
- Other factors such as HGF, FGF-7, EG-VEGF, VEGF, and β-NGF were also detected at varying levels.
Cytokines (average concentrations):
- Immunomodulatory cytokines: RANTES (551 pg/mL), IL-6R (53.3 pg/mL), IL-16 (8.7 pg/mL), IFN-γ (1.8 pg/mL)
- Pro-inflammatory cytokines: MCSF (930.8 pg/mL), MIP-1α (1.2 pg/mL)
- Anti-inflammatory cytokines: TNF-RI (191.6 pg/mL), TNF-RII (89.8 pg/mL), IL-1RA (58.8 pg/mL)
- Homeostatic cytokines: TIMP-2 (8,663.6 pg/mL), TIMP-1 (7,386.7 pg/mL)
- Wound healing cytokines: ICAM-1 (1,554.9 pg/mL), MCP-1 (119 pg/mL), G-CSF (91.6 pg/mL), GDF-15 (89.2 pg/mL)
- Regenerative cytokines: Growth hormone (GH) (31.1 pg/mL), GDNF (19.5 pg/mL)

Hyaluronic Acid:
- High concentrations of hyaluronic acid were observed, averaging 8.7 μg/mL.
Extracellular Vesicles:
- Nanoparticle tracking analysis identified an average of 17.4 billion particles/mL in the extracellular vesicle size range. Fluorescent staining confirmed an average of 4.18 billion membrane-bound extracellular vesicles per mL, indicative of true extracellular vesicles (such as exosomes).
Conclusion:
According to the researchers, Wharton’s Jelly contains a rich mixture of growth factors, cytokines, hyaluronic acid, and extracellular vesicles—all critical components in regenerative processes. They specifically highlighted the presence of growth factors (such as IGFBPs and TGF-α) involved in tissue regeneration and bone/cartilage formation. Immunomodulatory and anti-inflammatory cytokines were also present, potentially contributing to an optimal healing environment.
The researchers stated: “These results confirmed our hypothesis that growth factors, cytokines, hyaluronic acid, and extracellular vesicles are present in the formulated Wharton’s jelly. Several published basic science and preliminary clinical studies indicate that the combination of these factors may have added advantages for regenerative medicine applications.”
They also mentioned the potential superiority of Wharton’s Jelly compared with other biologics, due to the higher levels of critical regenerative components. For example, Wharton’s Jelly reportedly contains higher levels of cytokines and growth factors compared to products like platelet-rich plasma (PRP), amniotic fluid, or bone marrow aspirate concentrate.
They suggest these findings indicate promising expanded applications for regenerative medicine, although further clinical studies are warranted to confirm safety and efficacy.
Limitations:
- The assay kits used could detect only a limited set of growth factors and cytokines (40 each), meaning other potentially important factors were not measured.
- Extracellular vesicle analysis likely included both exosomes and other microvesicles. Specific exosome markers should be confirmed by further immunoblotting studies.
- Other valuable matrix components known to be present in Wharton’s jelly, including collagen and glycosaminoglycans, were not analyzed and should be investigated in future studies.
Potential conflicts of interest were acknowledged, as two authors are consultants and two others own equity in BioIntegrate LLC, which funded the study.
Link to original study: https://pmc.ncbi.nlm.nih.gov/articles/PMC7017504/