Key Findings:
- ExoPTEN achieved 100% motor function recovery in spinal cord injury rats
- Proprietary siRNA loading allows for temporary suppression of the PTEN pathway, enabling neural regeneration
- Stem cell exosomes are easier from both a manufacturing and clinical perspective
As you know, modern medicine is a miracle that’s kept us on our feet for much longer than God intended, but it hasn’t solved everything. There are still several conditions with very few answers, particularly in neurology, such as spinal cord injury (SCI).
However, the world has been given a new tool in the medical arsenal: regenerative medicine, and an Israeli startup named NurExone Biologic believes they may have figured out how to harness it for SCI.


Their lead therapy candidate, ExoPTEN, combines elements of stem cell exosomes and gene therapy to promote neural repair without the downsides of live-cell therapies.
Based on promising preclinical results and a thorough review, the FDA granted ExoPTEN Orphan Drug Designation in 2023.
What’s Orphan Drug Designation? When a therapy shows early promise for a rare or underserved condition, the FDA can grant this status to accelerate its development. It offers commercial incentives, such as tax credits, fee waivers, and 7 years of market exclusivity upon approval.
So far, the company has restored motor function in the majority of SCI rat models. The preclinical findings were first published in ACS Nano, and they’re hoping to launch the first in-human trials in 2026.
We sat down with the CEO, Dr. Lior Shaltiel, to discuss what ExoPTEN is, the science behind it, and what’s next for NurExone.

NurExone Biologic’s Background:
NurExone Biologic is a young startup with roots from two leading Israeli universities: Tel Aviv University and the Technion in Haifa. Years ago, two professors from these colleges joined forces to focus on treatment for irreversible spinal cord injury.
They explored a variety of methods and found the most promising to be mesenchymal stem cell-derived exosomes loaded with siRNA targeting the PTEN protein. In 2020, together with serial entrepreneur Mr. Yoram Drucker, NurExone Biologic was formed to commercialize this technology. We have named our first therapy under development ExoPTEN.
My background is in chemical engineering, with a master’s in physiology and a PhD in pharmacology from LMU Munich. I completed a postdoc at Goethe University Frankfurt before returning to Israel in 2014 to work on liposomal drug delivery systems, and I joined NurExone in 2021 as CEO.
ExoPTEN is our first and lead therapy, and it has shown effectiveness in preclinical studies across several models, including spinal cord injury, optic nerve models, and facial nerve models.
What Is ExoPTEN, and How Does It Work?
ExoPTEN begins with exosomes, derived from mesenchymal stem cells (MSC-Exos). If you aren’t familiar, exosomes are tiny extracellular vesicles secreted by various cells. They carry lipids, proteins, and genetic materials, and it’s one of the ways cells communicate with each other. MSC-Exos show great promise for neurodegenerative, orthopedic, and other conditions due to their regenerative potential. We’ll touch more on them later in the interview.
Our MSC-Exos are then loaded with a specific type of small interfering RNA (siRNA) to enhance their therapeutic effect by temporarily silencing the PTEN gene. PTEN is part of the mTOR signaling pathway, which regulates how cells use energy, grow, and respond to their environment. PTEN’s role in mTOR is to act as the “brake” in cell growth and division. It’s well known by oncologists because it functions as a tumor suppressor, preventing cells from growing uncontrollably.
mTOR pathway at a glance:

ExoPTEN works by knocking down PTEN expression, increasing mTOR signaling, and thereby promoting cell growth and survival. In cancer, unchecked PTEN suppression can be problematic, but ExoPTEN temporarily and locally suppresses that gene, which is very useful for regeneration.
Additionally, MSC-Exos are known to reduce inflammation, which is crucial for neurological recovery because neurons struggle to regenerate when surrounded by inflammatory signals. Our research indicates that ExoPTEN initially reduces the inflammatory period, followed by promoting regeneration and repair through siRNA.
We believe we’ve discovered a synergistic, dual-action approach with our therapy.
“In short ExoPTEN is inhibiting the inhibitor”
Preclinical Results
Let’s start off with a visual from our most recent preclinical work, then we’ll go through how we got here. The video below compares the control group (no treatment) on the left with rats treated with ExoPTEN. Notice the hind leg movement; you can see a striking difference:
In 2019, the first work on loaded exosomes was published in ACS Nano, investigating ExoPTEN in rats with a complete spinal cord transection, one of the most severe injury models.
When ExoPTEN was administered intranasally for five consecutive days, the treated rats showed clear functional recovery, with an average BBB locomotor score of 7.8 ± 2.1 at week 8, compared to only 0.4 ± 0.1 in untreated controls.
What’s the BBB locomotor score? Basso, Beattie, and Bresnahan locomotor score (BBB) is a standardized 21-point scale used to assess motor function in rats after SCI.
0 = complete paralysis, 21 = normal gait.
Roughly 30% of the ExoPTEN-treated rats regained coordinated stepping (BBB ≥ 14), and MRI imaging revealed partial restoration of neural tissue continuity, accompanied by measurable electrophysiological recovery. Following this breakthrough, NurExone Biologic was formed, which has enabled us to transition ExoPTEN into a fully controlled environment to study varying dosages/administration, and so far, we’ve achieved even better efficacy.
In our most recent preclinical work, ExoPTEN was tested in both transection and compression models, bearing in mind that compression is more reflective of real-world SCI. We also increased the dosages this time.
- In the transection model, intranasal ExoPTEN produced approximately 50–70% improvement in motor function, consistent with earlier studies.
- When given intrathecally, the same effect was achieved.
- And in the compression model, every rat in the ExoPTEN group regained the ability to walk, the first time the company has seen a 100% recovery rate in any preclinical model.
To evaluate spinal cord regeneration, we used diffusion-tensor MRI to track water-molecule motion (fractional anisotropy) and confirmed that neural fibers were reconnecting across the lesion. Histology slides revealed reduced cyst formation and a more organized tissue structure. When stained for vascular markers, the treated cords also displayed larger, denser blood vessels, suggesting improved blood flow and support for neuronal repair. Here are the DTI results:
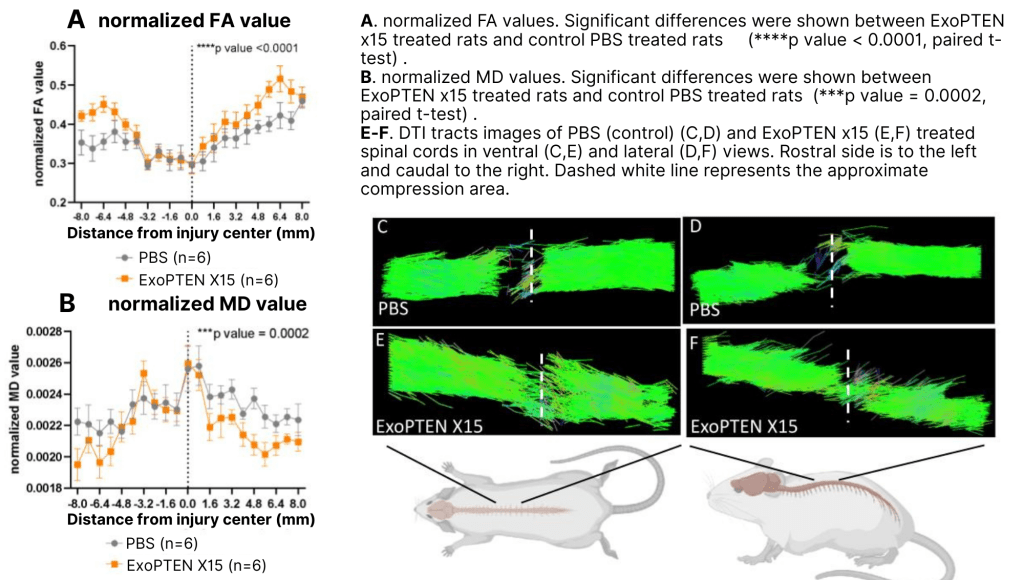
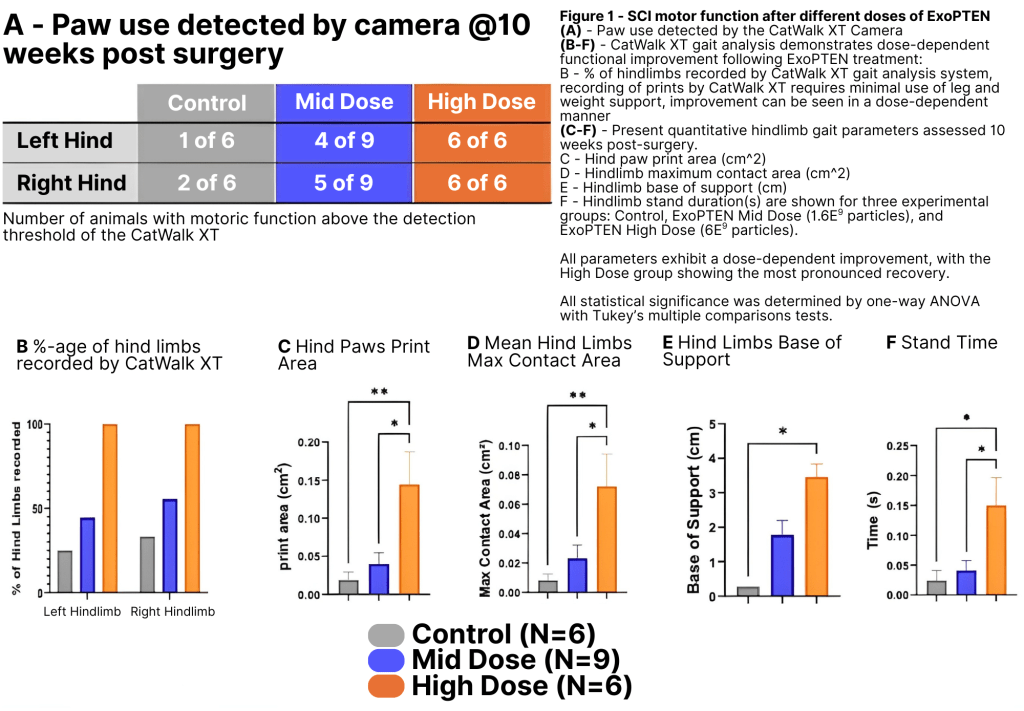
The most critical part, though, is the functional analysis: how well are rats recovering motor function? To investigate, the team used the CatWalk gait-analysis system, which records parameters such as paw placement, stride length, and inter-limb coordination as the animals walk across a glass platform. CatWalk removes observer bias from the older BBB locomotor scoring method, providing objective confirmation of true gait recovery. We found that 100% of the rats in the higher-dose group demonstrated measurable gait recovery, whereas only one animal in the untreated group exhibited minimal stepping. Here are those results:
Advantages of Exosomes vs Stem Cells
Clinical Advantages:
During our university research, it became clear that while stem cells can be very useful, much of their effect comes from what they secrete, specifically, the MSC-Exos. This led to the concept of “cell therapy without cells”. If injecting exosomes alone yields similar results, it’s much simpler than working with live cell therapies.
Why? For one, MSC-Exos allow for an allogeneic, off-the-shelf therapy. In traditional cell therapy, allogeneic products carry a risk of rejection, and autologous products can be invasive or costly (or both). One batch of MSC-Exos could potentially treat several patients without these downsides.
Also, MSC-Exos, even without siRNA, have a variety of innate therapeutic advantages. As described above, they’ve been shown to calm inflammatory signaling and naturally home in on those inflammation sites, acting like guided missiles without the need for engineering. They’re considered a nanomedicine, ranging from about 80 to 100 nanometers, similar in size to liposomes, but unlike liposomes, MSC-Exos can cross the blood-brain barrier to reach the CNS. We also tested this by labeling MSC-Exos with gold nanoparticles (a CT contrast agent) and confirmed their migration into the CNS.
Finally, MSC-Exos are easier to handle on the clinical end. Both stem cells and MSC-Exos are sensitive to freeze-thaw cycles and remain stable around –80 °C; however, MSC-Exos can survive at –20 °C for short periods. That flexibility is particularly important for smaller clinics that may not have access to ultra-low freezers.
I believe they represent the ultimate drug-delivery system for our indications.
Manufacturing Advantages:
The production process is also less complex. If stem cells are the final product, you’re going to need large bioreactors, upwards of 20,000 liters, to continuously grow and expand these cells.
With MSC-Exos, the stem cells act as tiny factories that secrete the therapeutic product. By perfusing the bioreactor and repeatedly harvesting that exosome-rich medium, we can achieve production with only small-scale, liter-sized systems. From an operational standpoint, this is a huge advantage.
Lastly, some companies have developed lyophilized MSC-Exos, available in powder form that can be reconstituted before injection to extend shelf life. We’re laser-focused on the production, loading technique, and clinical translation of our therapy, but in the future, this may become a reality.
Regen Report – Imagine some time in the future, your spinal cord is injured in an accident, and you simply head to Walgreens for a quick round of ExoPTEN, or an Amazon drone delivers it to your home. It sounds like science fiction, but so did antibiotics for strep throat just a few generations ago.
What’s Next?
We just announced plans for our first US commercial manufacturing facility in Indianapolis, and with demonstrated preclinical results in hand, the next big goal is to launch the first human trials. We aim for IND submission in the second half of 2026, and then we’ll open centers for the Phase I/IIa study.
The trial will likely investigate these endpoints:
- Reduction of time in the trauma center following SCI
- Sensory improvements
- Biomarkers
- Impacts of combining ExoPTEN with neurorehabilitation
On the first endpoint, reducing time spent in the trauma center not only provides significant financial relief for patients but also for hospitals, as each day is extremely expensive. This could also free up beds for other patients in need.
ExoPTEN may not be sufficient for chronic SCI patients. However, if we can prove it for acute SCI, we hope to be able to combine neural stem cell therapy with ExoPTEN to address chronic SCI as well.
Because Orphan Drug Designation is tied to a single indication, our focus is primarily on SCI. However, if the trials are successful, we imagine ExoPTEN could also help a variety of other neurological conditions.
Of course, it’s very early, but we think there’s hope for many people suffering. Regenerative medicine is on the horizon.
Special thanks to Dr. Lior Shaltiel for the interview!